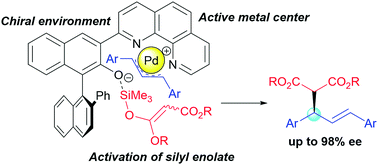
Design of bifunctional chiral phenanthroline ligand with Lewis basic site for palladium-catalyzed asymmetric allylic substitution - Chemical Communications (RSC Publishing)

SYNTHESIS AND STRUCTURE OF THE LINEAR BIMETALLIC ACETATE-PHENANTHROLINE COORDINATION POLYMER BASED ON PALLADIUM(II) AND NICKEL(II) IN A 2:1 METAL-TO-METAL RATIO | Journal of Structural Chemistry

Palladium(II) complexes of 1,10-phenanthroline: Synthesis and X-ray crystal structure determination - ScienceDirect
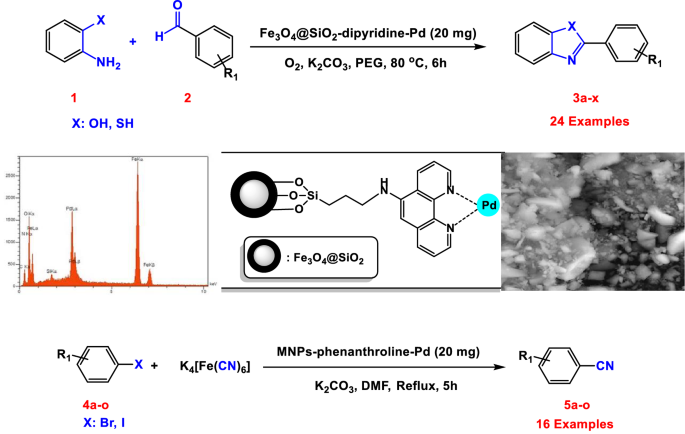
Palladium (II) Complex Supported on Magnetic Nanoparticles Modified with Phenanthroline: A Highly Active Reusable Nanocatalyst for the Synthesis of Benzoxazoles, Benzothiazoles and Cyanation of Aryl Halides | Catalysis Letters
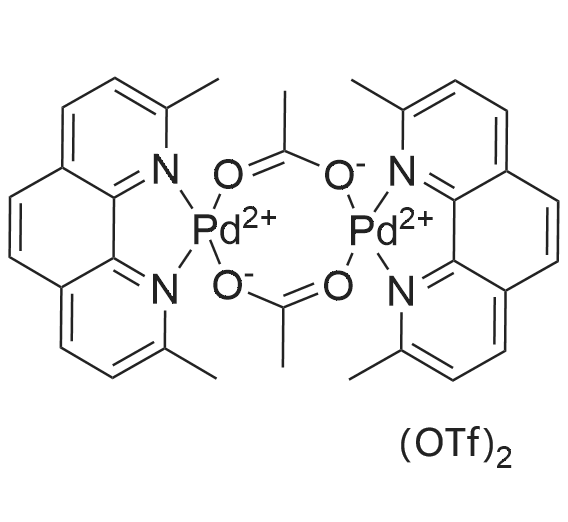
959698-20-5|Acetato(2,9-dimethyl-1,10-phenanthroline)palladium(II) dimer bis(trifluoromethanesulfonate)| Ambeed

Ligands with 1,10-phenanthroline scaffold for highly regioselective iron-catalyzed alkene hydrosilylation | Nature Communications

Synthesis, structures, photophysical properties, and catalytic characteristics of 2,9‐dimesityl‐1,10‐phenanthroline (dmesp) transition metal complexes - Cetin - 2020 - Journal of Polymer Science - Wiley Online Library
![PDF] Friedel–Crafts-Type Alkylation of Indoles in Water Using Amphiphilic Resin-Supported 1,10-Phenanthroline–Palladium Complex under Aerobic Conditions | Semantic Scholar PDF] Friedel–Crafts-Type Alkylation of Indoles in Water Using Amphiphilic Resin-Supported 1,10-Phenanthroline–Palladium Complex under Aerobic Conditions | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e39d732204017fe9546610084a4af86b6a3a9bdd/3-Table1-1.png)
PDF] Friedel–Crafts-Type Alkylation of Indoles in Water Using Amphiphilic Resin-Supported 1,10-Phenanthroline–Palladium Complex under Aerobic Conditions | Semantic Scholar

2-Hydroxy-1,10-phenanthroline vs 1,10-Phenanthroline: Significant Ligand Acceleration Effects in the Palladium-Catalyzed Oxidative Heck Reaction of Arenes | Organic Letters

US6281382B1 - Palladium phenanthroline acetate catalyst and a method of oxidizing side-chains of alkylbenzenes with catalyst - Google Patents

2-Hydroxy-1,10-phenanthroline vs 1,10-Phenanthroline: Significant Ligand Acceleration Effects in the Palladium-Catalyzed Oxidative Heck Reaction of Arenes | Organic Letters
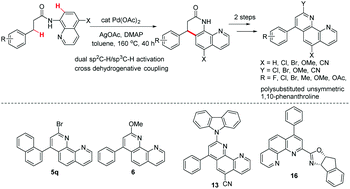
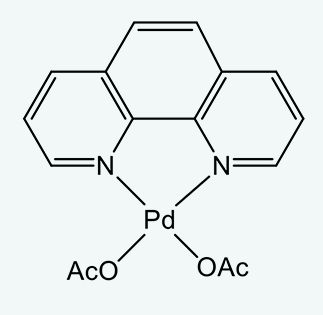
![Palladium Compounds [C-H Activation] | Tokyo Chemical Industry (India) Pvt. Ltd. Palladium Compounds [C-H Activation] | Tokyo Chemical Industry (India) Pvt. Ltd.](https://www.tcichemicals.com/medias/B5400.jpg?context=bWFzdGVyfHJvb3R8MzgzOTJ8aW1hZ2UvanBlZ3xoYzcvaDA5Lzg5MjkxOTk2NTI4OTQvQjU0MDAuanBnfDIwMDg1ZmRkYTE3ZTc1OTU4OWFmNDhhMzI3ZTEzODliNjI5MDBiZTUwODM1MTk1MTc3MGE0ZDQ4ZTk0ZmNiZTQ)